how many neutrons are in silicon|Atomic and nuclear properties of silicon (Si) : Baguio Mar 23, 2023 Board Exam Results 2024: Find here Results of Board Exams, UP Board, Bihar Board, Rajasthan Board, MP Board and other 10th and 12th exam results.Download videos online at high-quality from Facebook, TikTok, Instagram and more in seconds using our URL video downloader. All formats are supported!
PH0 · Silicon – expert written, user friendly element information
PH1 · Silicon (Si)
PH2 · Silicon
PH3 · Protons, Neutrons, Electrons for Silicon (Si, Si4+, Si4
PH4 · Protons Neutrons & Electrons of All Elements (List
PH5 · Chemical Elements.com
PH6 · Atomic and nuclear properties of silicon (Si)
PH7 · An Atomic Description of Silicon: The Silicon Molecule
Home About BulSU Academics Student Life Research, Extension & Innovation University News. August 2024 Archives. August 7, 2024. Launching of BulSU Hymn and March (Tagalog ver) ARCHIVES THIS YEAR. January May August. ARCHIVE BY YEAR. Contact Us. Guinhawa, City of Malolos, Bulacan (044) 919-7800.
how many neutrons are in silicon*******Naturally occurring silicon is composed of three stable isotopes, 28Si (92.23%), 29Si (4.67%), and 30Si (3.10%). Silicon-28 is composed of . Neutron Number and Mass Number of Silicon. Mass numbers of typical isotopes of Silicon are 28; 29; 30. The total number of neutrons in the nucleus of an .Silicon makes up 27.7% of the Earth’s crust by mass and is the second most abundant element (oxygen is the first). It does not occur uncombined in nature but occurs chiefly .
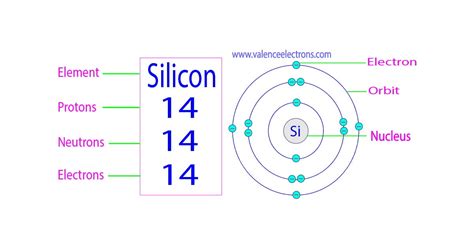
Mar 23, 2023 Atomic and nuclear properties of silicon (Si) Mar 23, 2023 Silicon has fourteen protons and fourteen neutrons in its nucleus, and fourteen electrons in three shells. It is located in group fourteen, period three and block p of the periodic table. .
Quantity: Value: Units: Value: Units: Atomic number: 14 : Atomic mass: 28.0855(3) g mole-1: Specific gravity: 2.329 : g cm-3: Mean excitation energy: 173.0: eV .Silicon is the second most abundant element in our planet’s crust. Oxygen (47.3%) and silicon (27.7%) together make up 75% of the weight of Earth’s crust. Most of the crust’s silicon exists as silicon dioxide; we are .
Number of Neutrons: 14. Classification: Metalloid. Crystal Structure: Cubic. Density @ 293 K: 2.329 g/cm 3. Color: grey. Atomic Structure. Number of Energy Levels: 3. First Energy Level: 2. Second Energy Level: 8. Third . Learn how the silicon atom, the basic unit of silicon, is involved in the photovoltaic effect, the conversion of solar energy to electricity. The silicon atom has 14 electrons, of which four are valence .
For all atoms with no charge, the number of electrons is equal to the number of protons. number of electrons = 30 number of electrons = 30. The mass number, 65, is the sum of the protons and the neutrons. To find the number of neutrons, subtract the number of protons from the mass number. number of neutrons = 65 − 30 = 35 number of neutrons . Therefore, Number of neutrons in Silicon = 28 – 14 = 14. As the neutrons and protons are located in the nucleus of an atom, the nucleus of Silicon can now be drawn as follows: . How many shells .
An Atomic Description of Silicon. The four electrons that orbit the nucleus in the outermost or "valence" energy level are given to, accepted from or shared with other atoms. The electrons orbit the .
Name of the isotope: Silicon-28; Si-28 Symbol: 28 Si or 2814 Si Mass number A: 28 (= number of nucleons) Atomic number Z: 14 (= number of protons) Neutrons N: 14 Isotopic mass: 27.976926535 (3) u ( atomic weight of Silicon-28) Nuclide mass: 27.9692465 u (calculated nuclear mass without electrons) Mass excess: -21.49279 MeV Mass defect: . Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) ( + 1) and a mass of 1 atomic mass unit (amu) ( amu), which is about 1.67 ×10−27 1.67 × 10 − 27 kilograms. Together with neutrons, they make up virtually all of the mass of an atom. Atomic mass of Silicon is 28.0855 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or .how many neutrons are in silicon Atomic and nuclear properties of silicon (Si) Silicates. Silicon is most commonly found in silicate compounds. Silica is the one stable oxide of silicon, and has the empirical formula SiO 2. Silica is not a silicon atom with two double bonds to two oxygen atoms. Silica is composed of one silicon atom with four single bonds to four oxygen molecules (Figure 2).
If an element has 12 protons and 17 neutrons, how many electrons does it have? How many protons, electrons, and neutrons are in the following isotopes. (a) Uranium-235 (b) Hydrogen-3 (c) Silicon-29; How many protons, neutrons, and electrons are in 3316S? How many protons, neutrons, and electrons are in chromium-53?how many neutrons are in siliconSilicon (14 Si) has 23 known isotopes, with mass numbers ranging from 22 to 44. 28 Si (the most abundant isotope, at 92.23%), 29 Si (4.67%), and 30 Si (3.1%) are stable. The longest-lived radioisotope is 32 Si, which is produced by cosmic ray spallation of argon.Its half-life has been determined to be approximately 150 years (with decay energy 0.21 .Quantity: Value: Units: Value: Units: Atomic number: 14 : Atomic mass: 28.0855(3) g mole-1: Specific gravity: 2.329 : g cm-3: Mean excitation energy: 173.0: eV .Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in .
Mass Number = # of Protons + # of Neutrons. Mass Number = 1 + 2. Therefore, this particular atom of hydrogen will have a mass number of 3. Note that the mass number calculated in Example 2.4.1 2.4. 1 does not .We would like to show you a description here but the site won’t allow us.
Si I Ground State 1s 2 2s 2 2p 6 3s 2 3p 2 3 P 0 Ionization energy 65747.76 cm-1 (8.15168 eV) Ref. MKMD94 Si II Ground State 1s 2 2s 2 2p 6 3s 2 3p 2 P° 1 / 2 Ionization energy 131838.14 cm-1 (16.34584 eV) Ref. MZ83-1 (16.34584 eV) Ref. MZ83
The silicon-29 isotope is used extensively in Nuclear Magnetic Resonance or NMR spectroscopy. It has 14 electrons, 14 protons and 15 neutrons.It is produced in the silicon-burning process from fusion of alpha particles and is the heaviest stable nuclide with equal proton and neutron numbers. Calcium-42 is composed of 20 protons, 22 neutrons, and 20 electrons. Calcium-43 is composed of 20 protons, 23 neutrons, and 20 electrons.

So we can put in a 12. The atomic number was six, right here. So we put in a six. Plus the number of neutrons. Plus the number of neutrons. So the number of neutrons is just equal to .How many Neutrons and electrons does silicon have? Silicon has 14 electrons and 14 neutrons. How many electrons does silicon gain or lose? Silicon gains 4 electrons. Trending Questions .
Key Features Make iTop Easy Desktop Stand Out Organize your computer desktop Desktop organization can be automated and totally free. iTop Easy Desktop improves its Type-based Rules, supporting grouping photos, shortcuts, documents as well as system icons into category boxes. Meanwhile, boxes can be numbered, titled, and colored. .
how many neutrons are in silicon|Atomic and nuclear properties of silicon (Si)